
A-Level化学考试辅导:知识点总结
A-Level化学有哪些知识点需要掌握?知识点那么多我要怎么复习才能高效呢?今天留学生辅导老师给大家说下,alevel考试辅导中,平衡知识的知识点复习总结。

Chemical Equilibria Revision
化学平衡知识点复习概要
01可逆反应
Reversible Reactions
(1)可逆反应
示意图如下A reversible reaction is one where there is a forward and backward reaction occurring:
![]()
双箭头含义The double arrow signifies a reversible reaction.
动态平衡:正向和逆向反应速率相等的时候If in the above reaction the concentrations of A,B,C,D do not change, although the reaction is still in progress, then the forward rate must equal the backward rate. A situation known as dynamic equilibrium has been reached.
(2)平衡常数Equilibrium Constants
所有的平衡反应的性质都可以用浓度平衡常数Kc来表示。Any dynamic equilibrium can be described in terms of its equilibrium constant,Kc.
The equilibrium constant is the product of the molar concentrations of the products raised to the power of its coefficient in the stoichiometric equation,divided by the product of molar concentrations of the reactants,each raised to the power of its coefficient in the stoichiometric equation.
具体方程关系如下:
So for the reaction:
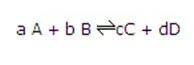
The equilibrium constant is given by:
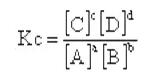
Where [ ] represents the concentration of the species in moldm-3.
对于由气态物质组成的反应来说, 可以用气体的分压来表示反应的平衡常数. For gaseous systems, we use Kp instead of Kc. Here, the species are shown in the equilibrium equation in terms of their partial pressures. (In a mixture of gases, the proportion of the total pressure due to a particular gas is dependent on its mole fraction).
关系如下: Kp=Kc*(RT)△n
(△n=反应生成的物质的量-反应物的物质的量)
02勒夏特列原理Le Chateliers Principle
定义含义Le Chateliers principle states:
平衡的移动总是向着逆转反应影响的方向。The position of the equilibrium of a system changes to minimise the effect of any imposed change in conditions.
This principle applies to any reaction that is in equilibrium.
(1)物质浓度变化对于平衡的影响The effect of concentration changes on equilibrium
改变反应物或者是生成物的浓度,不会影响这个反应的平衡常数,但是会引起平衡移动。Changing concentration of a reactant or product does not change the numerical value of the equilibrium constant,but it does change the position of the equilibrium.
In general,the position of the equilibrium is shifted towards the right if the concentration of a reactant is increased or to the left if the concentration of a product is increased.
At the start,when the change is made,the mixture is not at equilibrium, but equilibrium is eventually restored.
(2)压强变化对于平衡的影响The effect of pressure changes on equilibrium
对于前后气体分子个数不相等的反应,压强或者是体积变化也不会改变反应的平衡常数,但是会引起平衡的移动。For a reaction involving gases, altering the pressure may cause a change in the position of the equilibrium.
若反应后气体分子数增多,增大压强,平衡向着逆向移动。For a reaction where there is an increase in the number of moles from reactants to products, increasing the pressure moves the equilibrium to the left.
若反应前后气体分子数减少,增大压强,平衡向着正向移动。Where there is a decrease in the number of moles from reactants to products,increasing the pressure moves the equilibrium to the right.The equilibrium constant remains the same.
(3)温度变化对于平衡的影响The effect of temperature changes on equilibrium
The change that takes place when temperature is changed depends upon whether the forward reaction is exothermic or endothermic.
If the forward reaction is exothermic then the backward one is endothermic.
吸热反应,升温,平衡向正向移动。If the temperature is increased, the equilibrium moves to the left, since an endothermic reaction will tend to reduce the temperature.
放热反应,升温,平衡向逆向移动。Conversely, if the temperature is decreased then the equilibrium, moves to the right.
(4)催化剂/稀有气体对于平衡的影响The effects of catalysts on equilibrium
没有影响!A catalyst or addition of noble gas has no effect on the position of the equilibrium. However, a catalyst does increase the rate of both the forward and backward reactions, decreasing the time taken to reach equilibrium.
凡来源标注“考而思”均为考而思原创文章,版权均属考而思教育所以,任何媒体、网站或个人不得转载,否则追究法律责任。

kaoersi03















